Pyrolysis, Cracking and Hydrogen - Gashole plugged - on powering an engine
The movie "Gashole" completely misses the mark. Villifying the oil industry is not the answer to the thing WE ALL want to continue to use - our personal automobiles !! We need to just change from using oil based and other fossil fuels of the petrochemical (oil) industry
The web is littered with sites all claiming to be able to give you great fuel efficiency by using Hydrogen. Some are pushing "HHO" mixed gas systems, some do not know the difference in separated gas hydrogen generators and oxy-hydrogen gas generation, and ALL of them do not understand the science of what is actually occurring in the combustion chamber of the engine.
The fact is neither - and NONE - of these fuels alone as sold as solutions can be effective in maximum sense on today's fuel injected, computer controlled engines and none of these vendors have done research on how to do this - except us at hydrogenpowercentra.com and thus here is a short primer on what this is all about.
Pyrolysis: py·rol·y·sis (p -r l -s s). n. Decomposition or transformation of a compound caused by heat. This is a proper Chemistry term and definition of the field.
The pic is a schematic of a process of petrol-chemical distillate "cracking"
In the oil - or petro-chemical industry, they have made up and given the name of "Cracking" as the process where covalent bonds are broken in hydrocarbons to produce smaller hydrocarbons.
Cracking is the name given by the petro-chemical industry to breaking up large hydrocarbon molecules into smaller and more useful bits. This is achieved by using high pressures and temperatures without a catalyst, or lower temperatures and pressures in the presence of a catalyst.
The common thread to all "cracking" is HEAT and PRESSURE
"Cracking" is NOT a Chemistry term proper. It is in FACT, simply pyrolysis performed under PRESSURE.
What does this mean? It means you need to know more about the oil and refining industry to understand it.
In the late 19th century, crude oil, as recovered from the ground, could be distilled to separate the liquid materials by their boiling points. Remember that crude oil is a mixture of thousands of individual chemical compounds, ranging from very low molecular weight hydrocarbons such as propane (C3H8) to individual hydrocarbons with 30 or more carbon atoms.
In fact when the oil industry first started refining crude oil - to replace "Whale" oil; "gasoline" was largely burned off, and/or given away to those who wanted it and were close to the refining source.
These early refiners discarded the low boiling fractions, the part we now call gasoline, and recovered the more valuable , higher boiling, kerosene for lighting and heating. But the internal combustion engine required a lower boiling fuel than kerosene because the fuel had to vaporize in the engine cylinder, and electrical service replaced much of the demand for kerosene.
THIS is what spurred the entire auto industry and even the invention and development of the Otto cycle (four cycle) Internal combustion engine. Cheap, largely FREE fuel that was actually cheaper and "easier" than feeding and caring for a horse. Wow . . . but it is the truth.
So turn of the last century chemists developed the refinery practice of "cracking"; heating the crude to very high temperatures in the absence of air. They found they could produce additional amounts of the gasoline fractions by what seemed to be a process of rupturing chemical bonds to get smaller molecules.
The fact is, pyrolysis will occur in the combustion chamber of an internal combustion engine, and early patents like 2.407,478 and it's alleged buy up of it by Shell Oil company - who basically admitted as much, and history, movie reels by Shell and witnesses seem to bear as much of a 1947 Studebaker achieving 149.5 miles per gallon, are strong indications that "cracking" was and could be occurring under heat and pressure in a combustion chamber of an engine.
Patent 2,407,478 was for a device that injected (more like provided) steam into the engine air intake, and guess - what - that steam - water vapor in the excited molecular state broke into it's constituent parts of Hydrogen and Oxygen where the Hydrogen was immediately burned in a MORE explosive fashion to give the seemingly "magical" cooling extra BTU which produced the extra miles per gallon and reduced knock.
Another patent several years later, 4,177,779 around the time of the American "energy crisis" in 1979, proved a 200 mile per gallon vehicle (with a Ford 351 V-8) was possible using vaporized gasoline feed to the engine using a gaseous gas carburetor - aka an air-gas regulator. The inventor would not sell to the oil companies and suspicions are his untimely demise in his car in the desert of a drug over-dose was not as accidental as one might think.
The early patent - 2,407,478 was clearly pyrolysis at work - direct steam injection. The later 4,177,779 patent appears to be also as it is alleged the Inventor used a crude form of water injection to keep detonation to minimum.
Are the oil companies tying to keep these kinds of devices off the market? Certainly it would throw the refining Libra Pens out of whack. You need to see our movie "Gashole Plugged" which explains why it is NOT the oil industry trying to keep aything off the market -and in fact the oil industry is in a production trap now it cannot escape.
Not only Gasoline and Diesel comes from crude oil. AND all of the by-products must be disposed of somewhere in the marketplace.
However, NOWHERE in the refining equation is found Hydrogen as a direct petroleum distillate. It is in some cases a minor by-product - used to further refine more of the crude equation - but it is NOT primary constituent out-put of refining oil.
Everyone wants to claim there is a great conspiracy to prevent Hydrogen use in an Internal combustion engine as a wide spread motor fuel. It is not so - it is a logistics nightmare of safety, design, balance of use and proportion to potable water for consumption of animal and plant life on the planet. First, putting that above aside, you need to get enough of the gas to feed the engine for the size.
Second, for Hydrogen since it's best ratio is around 32 to 1, you need to pack enough air in to get a good bang for the buck.
Third, since Hydrogen is such a small diatomic atom, it can get right past the compression rings and collect in the crankcase, where it won't explode, it will just light the oil spray in the engine on fire. That is not a problem in any dangerous way, it just leaves a coke pile in the lifter valley eventually.
Fourth to make enough Hydrogen to power a Chevy 350 the Hydrogen generator needed is sizeable. That would be quite a bit of DC power needed to make that much gas per minute, and that does not mention the speed of the water-based fuel stock consumption that needs to happen to to make it all keep going.
Finally since when Hydrogen burns it turns back to water vapor, - steam - you need an extra hot spark and extra tough spark plug and ignition system to take that kind of hot spark for direct pyrolysis or water based fuel stock - but it can be done. (A major spark plug manufacturer has patented one by the way)
But this "cracking" process also found that the hydrocarbons produced in the cracking process had no better quality; they knocked, as much as the fractions taken from the uncracked crude. They had learned that branched chain hydrocarbons improved fuel performance and the early fuel engineers developed an experimental measurement of fuel quality based on knocking called octane number.
Analysis of the cracking results showed that these cracked fuels consisted of about the same percentage of "branched-chain" hydrocarbons as the uncracked material. For example, the hexanes present (C6H14), were no more likely to have branches in the links among the six carbons than be a straight, linear chain.
The reduction of engine compression ratio in the Model T reflected the declining quality and supply of gasoline in the World War I era.
With a precariously short lifespan for U.S. petroleum reserves, how could the automotive industry avoid catastrophe? The first proof we see of an effort to address this was in Patent 2,407,478.
At first this issue of pyrolysis amazed scientists, Shell Oil bought the patent, performed lots of research on this fact and then discovered this would be catastrophe in the other direction - they would be BACK to not being able to get rid of enough gasoline - AGAIN
An oil shortage also meant that higher quality petroleum was in shorter supply, and lower volatility components were finding their way into the fuel markets. Using modern yardsticks, fuel of this era was in the 50 to 60 octane range and declining. As a result, even automobiles with lower compression ratios were experiencing more engine knock.
This knock has to do with low vapor points and auto ignition temperatures of the fuel.
The higher the compression ratio, the easier the fuel lit off. "Dieseling" is term we also call it today - an engine that runs - or runs on - due to the heat it already has.
Engines are complex systems that never approach chemical or thermal equilibrium. Automotive engineers make difficult calculations in which the heat and temperature and compression and cooling effects cannot be so readily simplified.
The fact is - one searches for the ideal fuel which will not auto ignite at a high heat and high pressure, but easily ignite and burn slow and cool for maximum power. Only Hydrogen fits this bill - BUT Hydrogen also mixes easily with all other fuels - both affecting there properties and influencing them to "behave" better. It is not unlike adding cooler water to very hot water and at some point - you will have COLD water - but it will still be WATER.
To get WATER to change states it needs to be cooled to 32 degrees F to be solid or ICE; or heated to 212 degrees F. we call steam. When water changes to it's vapor state - what we know as steam - the molecule is very excited - and the more the atoms are excited by heating - they tend to want to break their covalent bonds into their constituent diatomic atoms of Hydrogen and Oxygen. But it stays a molecule until a magical sliding scale of heat and pressure when it will "crack" too - but then explode almost immediately due that heat because Oxygen is present and has nothing else to re-act with to go else where.
BOOM - more power to the Gasoline or Diesel explosion in the combustion chamber and from there came your magical extra BTU.
Splitting water by Pyrolysis can be done at any temperature it is a liquid, but the amount of heat required increases the cooler the water molecule is and the state it is in according to it's energy level.
The petro-chemical industry today is built on the transportation industry and to disrupt the delicate balance of the amount of refining and distribution done by America's oil by-product industry of which Gasoline and Diesel is a large component - would throw things in to a terrible disarray. Today the sources of crude oil to refine are not able to keep up supply for the demand at prices which support a healthy economy. In fact it is damaging our American economy instead running up costs of everything beyond the disposable income levels of American citizens and small businesses.
An "inventor" William H. Richardson, a machinist by trade; stumbled across what any welder who has ever arc welded under water knows. Explosive gas are the bubbles which come off the arc. Richardson stumbled across the fact that when using a Carbon Arc - it produced a new gas that was a combination of Carbon Monoxide and Hydrogen. Carbon Monoxide (CO) is flammable and will burn - while Carbon Dioxide (CO2) does not and will actually extinguish a flame.
Richardson managed to get a patent on the process of carbon arcing underwater to produce a fueling GAS - patent 5,435,274 in 1993. He was granted 5 more after that all based on the same phenomenon. It was a fuel that even could work with an Oxygen sensor. An Oxygen sensor MUST have some Carbon in the combustion equation to be able to determine a "Hydrocarbon" air fuel ratio. Burning pure Hydrogen produces NONE as there is no Carbon - to be the carbon part of the hydrocarbon.
The Oxygen Sensor is what controls the computers in all modern fuel injected gasoline engines, and is beginning to appear on Diesel engines also.
Though Richardson was granted all these patents, he remains struggling and is little known. The problem is with his invention, it needs a carbon arc, and all it does is produce COH2 a stable gas the value of which is slightly higher BTU content than straight Hydrogen, but has no features that allow it to be commercially compatible to the petrochemical industry. it is more akin to artificial natural gas, and frankly no one needs that right now today.
An "Alkane" - (also known as a paraffin or saturated hydrocarbon) is a chemical compound that consists only of hydrogen and carbon atoms and are bonded exclusively by single bonds. That means Richardson's gas is - yes is not even an Alkane or a.k.a. a Hydrocarbon fuel.
The movie "Gashole Plugged" - you can find here - is something you need to see if you want to seriously save $4,000 or more per year - because if you are an average American - that is what you are spending at the very least on fuel for one automobile alone today.
Without explaining how, needless to say we have considered all of this. It ain't rocket science, but it is harder than you think.


 ,
, 

 ,
,  ,
,  ,
, 






 ,
,  ,
,  ,
,  ,
, 
 ,
,  ,
,  ,
, 


 ,
,  ,
, 
 ,
,  ,
, 



 ,
,  ,
,  ,
,  ,
,  ,
, 



 ,
, 
 ,
,  ,
,  ,
,  ,
, 
 ,
,  ,
, 

 ,
, 



 ,
, 
 ,
,  ,
, 
 ,
,  ,
,  ,
,  ,
, 









 ,
,  ,
, 






 ,
,  ,
,  ,
, 


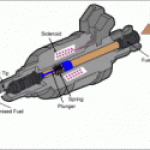











 ,
,  ,
,  ,
,  ,
, 


