Acetone, Octane and Hydrogen
You can bounce all over the web and find references to improving mileage and perfomance by adding Acetone to Gasoline. Others are all asking - does it work and is it safe. Yes and yes if you know what you are doing. 1 oz per gallon is about right - not more than two, unless you have really bad fuel, over four and benefits go down, and the effective price per gallon of gasoline you are causing goes up. At one ounce per gallon, you add about 10 cents to each gallon of gas - assuming a gallon of acetone is $13.00. BUT . . . it is more like $20 per gallon now.
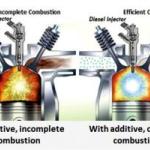
When Acetone burns in its proper stoichiometric mixture of about 4 to 1 it only produces CO2 and H2O, so it is a "non-polluting" fuel as we know it - but it still produces the greenhouse gas CO2. The truth is even as small an amount of Acetone as 1 oz per gallon will greatly increase the flammability of gasoline, but the speed at which it will burn or even pre-ignite may produce unpredictable results, such as detonation or ping anyway due to pre-ignition, or it may solve it. The fuel mix will definitely have more "strength" - that is BTU /per gallon power to propel the vehicle, and if you do not have a fuel injection system that will normally automatically retard the timing when ping or knock from detonation is detected - you will hear it.
The truth is Acetone is the "hottest" solvent with the lowest vapor point, while Toluene is lower, and Xylene is a bit higher than both. Of the three as a gasoline additive, Xylene is likely to give the best results to remove knock or ping, but give the lowest BTU per gallon increase also. Toluene is in the middle and will produce hotter exhaust gases - and due its low burn rate, (slow flame speed) may actually exhaust the combustion chamber still burning. A new comer in the middle of Toulene and Xylene is called "Industrial Maintenance Coating Thinner", (IMCT) and it allows you to feel the extra BTU per gallon - the engine will have more easy cruise power. It smells like finger nail polish remover, and is recommended for where Toulene, Xylene, MEK (Methyl Ethyl Keytone) or VMP Naptha is called for.
IMCT is a petroleum distillate such as "paint thinner" also once called "white gas", and Ethyl Acetate, an "Ester" mixed together. Ethyl acetate is used primarily as a solvent and dilutent, being favored because of its low cost, low toxicity, and agreeable odor. For example, it is commonly used to clean circuit boards and in some nail varnish removers (acetone and acetonitrile are also used). Coffee beans and tea leaves are decaffeinated with this solvent. It is also used in paints as an activator or hardener. Ethyl acetate is present in confectionery, perfumes, and fruits. In perfumes, it evaporates quickly, leaving only the scent of the perfume on the skin.
Ethyl Acetate, an Ester is a molecule with very loose Hydrogen bonds, and in the real world, in Gasoline seems to give them up easily. IMCT when added to gasoline raises the vapor point, increases the BTU per gallon and thus gives the best effect of the three others above.
There are Summer and Winter blends of Gasoline in America, and in the summer gasoline has more ethanol typically blended into it as an Oxygenator. Blening in Ethanol does not cause ping or pollute as much BUT raises the vapor point and lowers the BTU per gallon, thus it would make a vehicle harder to start - and maybe impossible without something like Ether (starting fluid) - in colder weather.
So the Winter blend of gasoline has a lower vapor point, and this was tested on a chronic Vapor Locking engine - the carbureted SBC Chevy with cast iron intake manifold with wide open exhaust passage cross over and open (to the exhaust manifold) hot air riser in a Van configuration where air circulation is tightly restricted unless moving down a road forcing air to flow through the engine compartment.
Long story short - non-fuel injected, non-electronic carburetor engines cannot adjust the timing automatically to address ping and knock. California straight Winter blend Regular "el cheapo" "rot-gut" gas, pings and knocks and rattles terribly.
Temperature goes up, and it would vapor lock sitting at a stop light for three minutes or more or in short stop and go traffic famous in southern California . Adding 1 oz per gallon ICMT silences the ping and rattle of the valves, increases throttle response as though it were Premium gas and power, and the engine runs cooler, idles smoother at a higher RPM also, and does not vapor lock in stop and go traffic.
In the end it is a hit and miss proposition, and you would need to carefully experiment with the same fuel to find the happy medium - depending on the refinery and brand name manufacturer of the fuel you are using; and that is the truth.
All of them at best will only basically turn cheap regular into the Octane of "premium" gasoline, and is only then a few pennies per gallon cheaper than buying premium gasoline - at most stations in the first place. The sad truth is some premium gasoline is not really that after all and is a rip off - but you cannot prove it. There is no portable "Octane" tester to measure how they achieve the higher octane - is it only Alcohol added? Could be. Lower BTU per gallon but higher resistance to knock or detonation.
Vapor/air mixtures are flammable only over a limited range of vapor concentrations. This range is defined by the lower and upper flammability limits. Mixtures outside this range are described as, respectively, too "lean" or too "rich" for ignition. The flammability limits are best explained by an example; we chose the common hydrocarbon acetone here. At standard temperature and pressure (STP), the lower and upper limits are 2.6% and 12.8% by volume. A stoichiometric mix of vapor and air contains just enough oxygen to burn all the vapor, with nothing left over. The stoichiometric ratio for acetone at STP is 5.0%. Note that this value is about twice the lower flammability limit and about one-half the upper limit.
The chemical formula for acetone is: C2H3COH3 and the balanced oxidation reaction for complete (stoichiometric) combustion is:
C2H3COH3 + 4O2 --> 3CO2 + 3H2O
This 4:1 ratio of oxygen molecules to acetone molecules is readily shown to be consistent with the stoichiometric amount of 5% by volume as stated above.
How it works to improve the octane and performance of gasoline - and yes even diesel fuel - is what this article is about.
The "octane" rating of fuel came about by using the resistance to knock [detonation] standard of - you guessed it "octane". Octane is a chemical fuel like heptane, pentane, propane, and butane. Octane is the eight hydrocarbon chain version in its laboratory grade. We have come to call it generically gasoline - the eight carbon chain hydrocarbon liquid. However in th family of liquids known as gasoline is a wide range from just above Kerosene to just under Naptha in the family of petroleum distillates..
Detonation is a fancy way to to say the fuel "auto ignited" before the desired time - also called "pre-ignition" Each fuel has an "autoignition temperature" which is the temperature the fuel will burst into flames on it's own at Standard Temperature and Pressure. Add Pressure and that temperature changes, which is why the modern internal combustion engine runs at all.
The Auto-Ignition Temperature - or the minimum temperature required to ignite a gas or vapor in air without a spark or flame being present - are indicated for some common fuels below:
Fuel or Chemical
Temperature
(oC)
(oF)
Acetone
465
869
Acetylene
305
581
Benzene
560
1040
Butane
420
788
Carbon
700
1292
Carbon monoxide
609
1128
Coal-tar oil
580
1076
Coke
700
1292
Cyclohexane
245
473
Diethyl ether
160
320
Ethane
515
959
Ethylene
490
914
Ehtyl Alcohol
365
689
Fuel Oil No.1
210
410
Fuel Oil No.2
256
494
Fuel Oil No.4
262
505
Heavy hydrocarbons
750
1382
Hydrogen
500
932
Gas oil
336
637
Gasoline
280
536
Gun Cotton
221
430
Kerosene
295
563
Isobutane
462
864
Isobutene
465
869
Isooctane
447
837
Isopentane
420
788
Isopropyl Alcohol
399
750
Light gas
600
1112
Light hydrocarbons
650
1202
Lignite - glow point
526
979
Methane (Natural Gas)
580
1076
Methyl Alcohol
385
725
Naphtha
550
1022
Neoheaxane
425
797
Neopentane
450
842
Nitro-glycerine
254
490
n-Butane
405
761
n-Heptane
215
419
n-Hexane
225
437
n-Octane
220
428
n-Pentane
260
500
n-Pentene
298
569
Petroleum
400
752
Production gas
750
1382
Propane
470
878
p-Xylene
530
986
Toluene
535
995
Wood
300
572
Xylene
463
867
The flammable (explosive) range is the range of a gas or vapor concentration that will burn or explode if an ignition source is introduced. Limiting concentrations are commonly called the lower explosive or flammable limit (LEL/LFL) and the upper explosive or flammable limit (UEL/UFL).
The stochiometric mixture is the one where it is just right to burn the fuel completely. For gasoline that is 14.7 to 1, but the noxious side pollutants also produced are intolerable to our air quality and do not go away but accumulate in the air - hence emission control sytems for gasoline powered engines..
Below the explosive or flammable limit the mixture is too lean to burn. Above the upper explosive or flammable limit the mixture is too rich to burn. The Auto-Ignition Temperature is not the same as Flash Point - The Flash Point indicates how easy a chemical may burn.
Note that Acetone has a high auto-igntion temperature as does Hydrogen and Natural Gas (methane)
Notice Octane and Gasoline
Now you do not need to be a genuis to see that some of these fuels are what we know as "gaseous gases" - that is they exist normally as a gas vapor at Standard Temperature and Pressure - STP - to simpifly things lets call it room temperature.
Many of the high auto-ignition temperature fuels have high evaporation rates - that means they turn to a vapor easily around room temperature, and will thus burn or explode if an ignition source is introduced. How dangerous they are normally depends on their molecular weight according to the ambient - or surrounding air - hydrogen is gone up and away fast, while propane is heavier and will linger near the ground.
So what happens when you add Acetone to gasoline is that you lower the explosive range while raising the autoignition temperature just slightly, AND the stoichiometric range increases from 14.7 to 1 a few points on each side and this gives you more power because the mixture will burn more completely and will not pre-ignite as easily - hence the knock goes away. In essence you raised both the explosive quality of the fuel, and raised the "octane rating"
People say Acetone has an Octane rating of 140 or 150 and this is likely true. Hydrogen has a laboratory Octane rating of 130, but the question becomes one of economy - and the heat and speed of the burn. At $13.00 per gallon, one cannot fuel their car completely with Acetone. If you did, the vapors are extremely dangerous with a stoichiometric mixture of 4 to 1 acetone vapor will explode very easily in lower vapor concentrations of LEL/LFL of 2 to 1 to 8 to 1, and it is heavy enough to lay low and explode. Also the straight Acetone burn is very hot and will burn valves and engine parts and coke the oil.
So at around the 1 ounce concentrations, at 10 cents per gallon, you can buy cheap gas and improve it's quality for less than buying premium gasoline. 2 ounces 20 cents and so on. More is not better - since it takes only a little bit more to surpass the UFL and defeat yourself. Also acetone is a very strong solvent - it is not "corrosive" as many sites seem to keep repeating. Corrosive means it attacks metal, and it does not - it dissolves man-made hydrocarbon materials like plastics and blended rubbers. so too much of it - in amounts over what it take to burn - might attack seals and diaphragms of your fueling system.
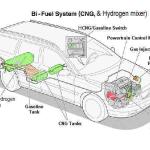
Hydrogen comes into the question because it is FREE when made on the fly, and introduced as a vapor into the air stream does th same thing in the combustion chamber except the problem is - while the burn range is very wide - from 17 to 1 to 75 to 1 - with 32 to 1 being ideal - if bi fueled with gasoline, hydrogen when it burns produces only water vapor, and must be introduced in a balanced amount to allow both fuels to burn completely as it extinguishes the burn with the water vapor it produces - or go to straight hydrogen fueling all together. We mean pure, sole, Hydrogen - NOT Oxy-Hydrogen gas - from splittng water based liquids and feeding BOTH the Oxygen and Hydrogen into the mixture. Some call it "HHO"
Hydrogen at STP is not a liquid and thus it requires a gaseous gas fueling system, and as it needs a 32 to 1 air fuel ratio - since you won't get about 15 to 1 naturally aspirated, you need to turbo or supercharge the engine.











 ,
,  ,
, 

 ,
,  ,
, 


 ,
, 
 ,
,  ,
,  ,
,  ,
, 
 ,
, 




 ,
, 





 ,
,  ,
, 

 ,
,  ,
,  ,
, 
 ,
,  ,
,  ,
,  ,
, 


 ,
,  ,
, 





 ,
,  ,
,  ,
, 


 ,
,  ,
,  ,
, 



 ,
,  ,
,  ,
,  ,
, 
 ,
,  ,
,  ,
,  ,
, 






 ,
,  ,
,  ,
,  ,
,  ,
, 







 ,
,  ,
, 




